Description
Manufactured by GE HealthCare
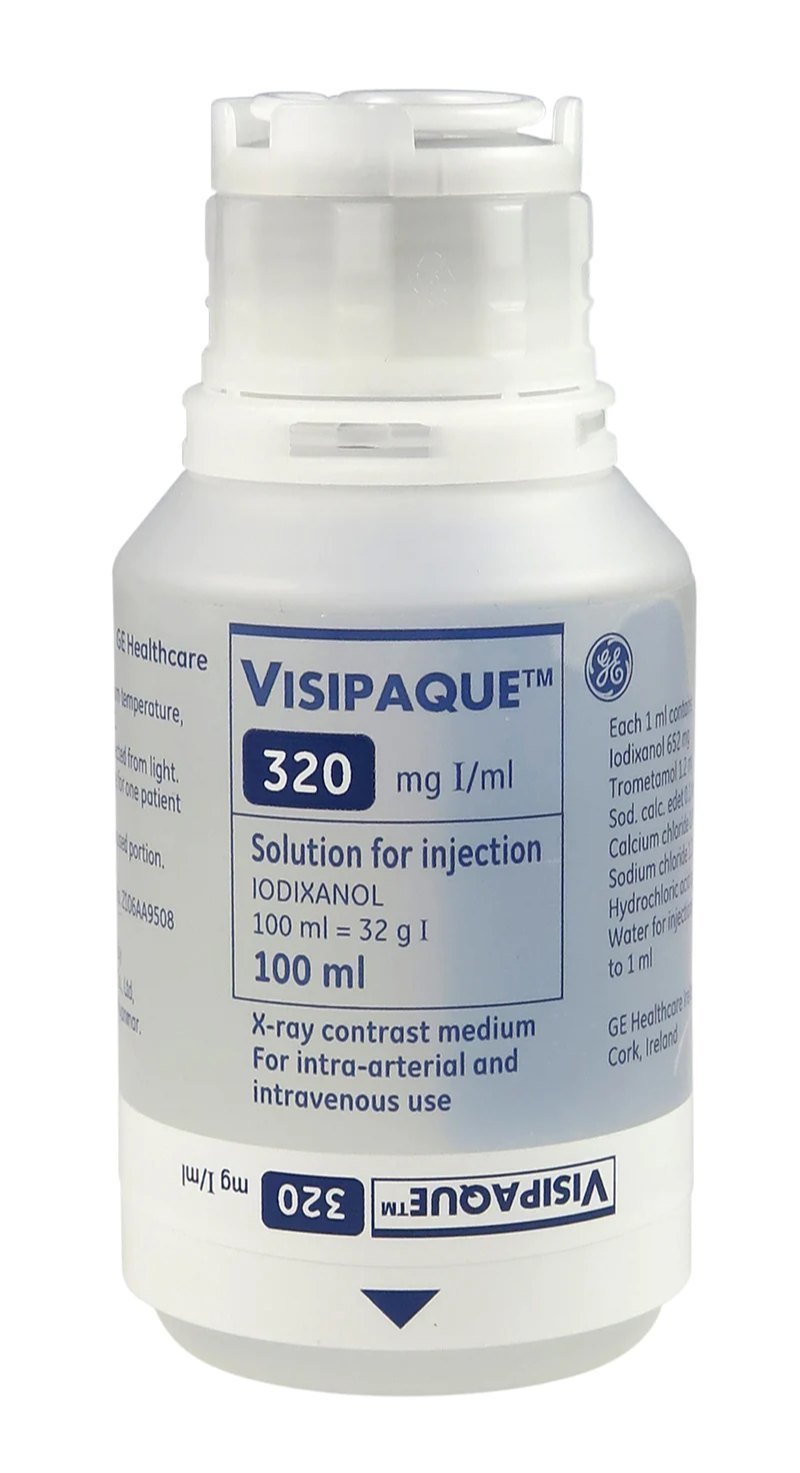
Visipaque™ is a non-ionic, dimeric iodinated contrast agent designed for intravascular use in diagnostic imaging procedures such as computed tomography (CT), angiography, urography, and other radiographic examinations. Developed by GE HealthCare, Visipaque stands out as the world’s first and only iso-osmolar contrast media (IOCM) with an osmolality equivalent to blood plasma, providing an advanced solution for minimizing contrast-induced adverse events while delivering high-quality diagnostic clarity.
Pharmaceutical Composition
Active Ingredient: Iodixanol (chemical name: 5-[acetyl(2,3-dihydroxypropyl)amino]-N,N′-bis[2,3-dihydroxypropyl]-2,4,6-triiodo-1,3-benzenedicarboxamide)
Formulation:
-
Available in concentrations of 270 mg I/mL and 320 mg I/mL
-
Supplied as a clear, colorless to pale yellow, sterile aqueous solution
-
pH: Approximately 6.8–7.6
-
Osmolality: ~290 mOsm/kg H2O (iso-osmolar to blood)
-
Viscosity: Increases with iodine concentration; important for injector compatibility
Key Clinical Advantages
✅ Iso-osmolar Formulation
Visipaque’s osmolality is similar to human plasma (about 290 mOsm/kg), making it significantly less likely to cause endothelial irritation, heat sensation, and fluid shifts compared to high- or low-osmolar contrast media (HOCM or LOCM). This makes it ideal for sensitive patient populations, including those with renal impairment, diabetes, or cardiovascular disease.
✅ Non-Ionic and Dimeric Molecular Structure
Visipaque’s dimeric structure results in higher iodine content per molecule while maintaining iso-osmolarity. Its non-ionic nature reduces the risk of adverse reactions, such as pain or allergic-like responses, often associated with ionic contrast agents.
✅ Superior Patient Tolerability
Clinical studies have demonstrated a lower incidence of injection-associated discomfort, nausea, and vomiting compared to some other non-ionic agents. It is particularly suited for patients at increased risk of contrast-induced nephropathy (CIN).
Indications for Use
Visipaque is indicated for intravascular administration in a wide range of adult and pediatric imaging applications, including:
-
Computed Tomography (CT):
-
Head, chest, abdomen, and pelvic scans
-
CT angiography (CTA) for vascular imaging
-
-
Angiography:
-
Cerebral, coronary, peripheral, and visceral vessels
-
Digital subtraction angiography (DSA)
-
-
Cardiac Imaging:
-
Left ventriculography and coronary angiography
-
-
Excretory Urography
-
Peripheral Venography and Arteriography
-
Pediatric Radiology:
-
Safe and effective across various age groups, including neonates
-
Dosing adjusted based on weight and indication
-
-
Intra-articular and body cavity contrast (off-label in some regions)
Safety Profile
Visipaque has been widely studied for its safety in both routine and high-risk patients. Benefits include:
-
Lower nephrotoxicity compared to LOCM in selected patients
-
Reduced heat and pain sensation during injection
-
Low incidence of hypersensitivity reactions
-
Fewer hemodynamic changes during coronary procedures
However, like all iodinated contrast agents, it carries potential risks including:
-
Contrast-induced acute kidney injury (CI-AKI)
-
Allergic-like reactions (rare, typically mild)
-
Delayed skin reactions
-
Thyroid dysfunction, especially in neonates or patients with thyroid disease
Contraindications:
-
Known hypersensitivity to iodixanol or any ingredient in the formulation
-
History of severe contrast media reactions (unless appropriately premedicated)
Packaging and Storage
-
Available in ready-to-use vials, prefilled syringes, and bottles
-
Common pack sizes: 50 mL, 100 mL, 200 mL
-
Storage: Store between 20°C–25°C (68°F–77°F); protect from freezing
-
Do not use if particulate matter or discoloration is observed
Why Choose Visipaque?
-
Enhanced diagnostic confidence with high image quality
-
Minimized patient discomfort through physiological osmolality
-
Broad clinical utility across all major imaging modalities
-
Backed by GE HealthCare’s global reputation in radiology innovation and patient safety
Regulatory Information
-
U.S. FDA Approved
-
Available in over 100 countries
-
Complies with European Medicines Agency (EMA) standards
Visipaque™: Designed for Clarity, Engineered for Safety.
When diagnostic precision matters, trust Visipaque from GE HealthCare—pioneering contrast media with patient-centered performance.




Reviews
There are no reviews yet.